BioDiamond
Benefits from BioDiamond Stents Family: Surface, Basic Steel and Design
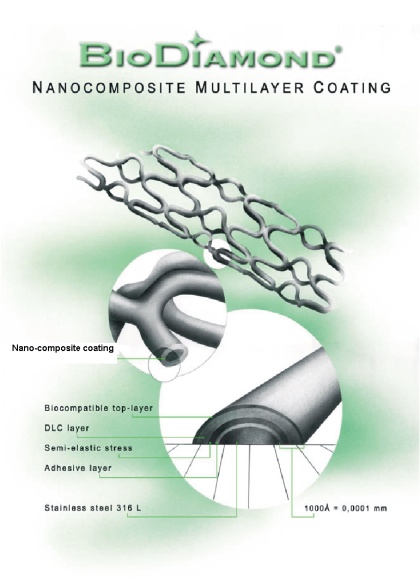
1. Multilayered nano-composite coating
BioDiamond coating presents ultra-thin (in scale of 1 hundred nano-meters) Multi Nano-Layered Carbon (MNLC) construction where each layer plays own role:
- 1st adhesive layer binds coating to stainless steel
- 2nd stress relaxation layer is responsible to relax any mechanical or thermal expansion stress developed between coating and steel. In case of any defects appeared on top layers this layer does not allow the defect growth through the whole coating up to steel.
- 3rd layer is Diamond-Like Carbon (DLC) layer responsible for good barrier against heavy metal ions penetration
- 4th biocompatible anti-thrombogenic top-layer, which comes in direct contact to the blood/tissue
Only BioDiamond technology (which is currently under international patenting procedure) allows high quality uniform coating inside the stent (overcoming Faraday┤s cage effect which prohibits to coat inner surface of stent by known sputtering/plasma approaches).
2. High flexibility + High stability: low re-coil and long-term radial stability under heart pulsing compression. Special steel features.
The required flexibility of a stent is always in contradictory to required mechanical stability (low re-coil). With stents of the "BioDiamond F" family, this contradictory is solved by the combination of "Flexible" and "Dense" segments (F- and D-segments) were F-segments are responsible for stent┤s flexibility and D-segments for high stability after expansion. The basic material of BioDiamond stents is stainless steel which in contrast to the most of products on the market retains sufficient part of elastic component ("real steel" behaviour). This feature is especially important for long-term radial stability because elastic component prevents accumulation of irreversible compression under billions cycles of heart beats.
3. Low injury - high precision mechanical structure
a) Special developed BioDiamond electropolishing process results in a specific profile of the wall thickness, which along with special design features allows the start of expansion from the middle of the stent (see attached Figure), avoiding "Thorn"-trauma of aorta┤s intima by "pins" located at the both ends of stent. It prevents also unpredictable movements of stent at start of expansion in lesion (because of small irregularities in wall thickness standard stent begins opening from the one of the ends which causes pressing of stent out of lesion in direction of this "first opening" end).
b) Thin profiled wall allows the use of high surface density of stent┤s elements (low opened surface) in BioDiamond stents because only at such thin profiled wall dilatation is possible at reasonable pressure at given high density of metal elements. It leads to the lower specific pressure (N/mm2) of elements on vessel wall at dilatation, i.e. to decrease of "Cutting" traumatic effect.
c) Developed electropolishing process gives highly reproducible precision structure, e.g. absolute deviation in wall thickness ▒ 5-8 Ám (other stents on the market measured by us, are electropolished with accuracy ▒ 15-25 Ám) which gives highly reproducible mechanical behaviour and clinical results.
4. Heavy Metal ion diffusion barrier
BioDiamond coating is safe barrier against elution of heavy metal ions from stainless steel to surrounding tissues and blood. This is very important issue because:
a) heavy metal ions are able to inhibit regulatory enzyme cascades
b) heavy metal ions are able to denature enzymes and proteins (generation of neoantigens, inhibition of enzymes etc.)
c) even in case when heavy metal atom still bound to the surface, it┤s non-compensated free surface valences may attack binding biomolecules and cause their damage and denaturation
d) heavy metal ions could initiate hyperproliferation of smooth muscle cells even in very low concentration
5. IN VITRO Studies: Highly biocompatible surface
In vitro studies by Dr. Klein of the University of Mainz and Dr. Kai Gutensohn of the University Hospital Eppendorf reveal that the surface of DLC stents is in contrast to 316 L stainless steel surfaces - highly biocompatible. In contrast to stainless steel the adhesion and activation of polymorphonuclear leukocytes and platelets is significantly reduced on DLC. Thus inflammatory processes are significantly reduced as well. As shown by flow cytometry the thrombocyte activity markers CD 62p and CD 63 are reduced in diamond-like coated stents compared with uncoated stents. The direct experiments on Total Occlusion (stent is expanded inside plastic tube through which the blood is circulating until total occlusion by thrombus) shows sufficient lower thrombogenecity of DLC in comparison with stainless steel.
The adherence of human umbilical endothelial cells is significantly improved on DLC in comparison to stainless steel.
Further factors for the highly biocompatible DLC stent surface can be explained by its low protein binding (shown by measuring the absorption of bovine serum albumin on DLC coated and uncoated sensor chips in a Biosensor ASI 1400 from ASI Company, Switzerland), extremely smooth surface (shown by scanning electron microscopy) and low release of cytotoxic and allergenic metal ions like nickel and chromium (shown by atomic absorption spectrometry in sera after implantation).
Dr. Gutensohn received the Prevention Prize of 2000 from the German Heart Association (Deutsche Herz Hilfe e.V.) for his ground-braking studies with DLC stents.
IN VITRO results can be shortly summarized as followed:
a) rapid proliferation of endothelial cells
b) non-activation of polymorphonuclear cells (no immune response)
c) strongly reduced activation of platelets and clotting factors (reduced risk of thrombosis)
d) strongly reduced serum proteins absorption, non-denaturation of proteins and enzymes (non immune response, non inhibition of enzymes, non presentation of neoantigens etc.)
e) non-recognition of foreign material like stainless steel 316 L by biosystems
f) non "Hyper-Encapsulation" of foreign material (restenosis) like in case of stainless steel 316 L
For further details please revert to attached papers.
6. Excellent clinical results. Comparison to other stents on the market
The most serious problem in modern intracoronary stenting is in-stent restenosis. Without any exception restenosis rates (defined as a 50 % reduction in lesion when compared to reference luminal diameter) of around 30 % are observed with uncoated metal stents in the six month follow-up after percutaneous transluminal coronary angioplasty. It is thought that restenosis is caused by the hyperplasia and apoptosis of smooth muscle cells. During activation polymorphonuclear leukocytes and platelets release growth hormones and interleukins, which supposedly cause the uncontrolled growth of smooth muscle cells. Thus a significant reduced restenosis rate should be expected with BioDiamond (DLC)stents.
Results from various clinical centers indicate that major adverse cardiac events (MACE) are significantly reduced with DLC stents in comparison to the state of the art during the clinical follow-up six months after the percutaneous transluminal coronary angioplasty (PTCA) including stent implantation. With 97 patients of the University Hospital Eppendorf no acute thrombosis or restenosis (the renarrowing of the successfully dilated segment by smooth muscle cell hyperplasia) could be observed in the 6 month follow-up. With 20 patients in the University Hospital St. Ekaterina in Bulgaria one subacute thrombosis was observed 1 month and two in-stent restenosis events 6 months after PTCA, of which one had to be treated by re-PTCA. With a non-randomized study group of 40 patients at the Evangelismos Hospital in Athens, Greece, one acute thrombosis was observed and seven patients presented with recurrent angina or a positive thallium scan in the angiography due to progression of the disease in arteries other than the stented vessels during the six month follow-up. None of these patients had repeat PTCA due to in-stent restenosis.
The first most detailed studies on BioDiamond stents were performed in the CHP Beauregard in Marseille, France, by Dr. P. Barragan. Table 1 compares the results of this study with 3 studies with uncoated and 2 studies with coated intracoronary stents. The results indicate that the major adverse cardiac events are significantly lower with the DLC stent in comparison to all other products. Furthermore a high portion 37 % - of the patients in the Barragan study were high risk (table 2), whereas the SOPHOR A and B studies with the BiodivYsio stent of Biocompatibles reveal a much lower percentage of these high risk patients. The BiodivYsio stent is coated with phosporylcholine and reveals the lowest MACE and restenosis rates, observed with stents so far. The results from Dr. Barragan indicate that the DLC stent is even better. Thirty-six of the patients of Dr. Barragan had undergone prior PTCA, with up to 3 stents already implanted and the occluded lesion to be treated with the DLC stent. In these patients restenosis rates are expected to be over 60 %. A further 17 patients had coronary artery bypass grafting with expected restenosis rates between 40 to 70 %. If one considers only de novo lesions and not these high risk patients, the rate of major adverse cardiac events is reduced to a surprisingly low 3.7 % with DLC stents (table 4).
TABLE 1: Data from six month clinical follow-up: Major Adverse Cardiac Events, MACE
Company |
Guidant |
Boston Sientific |
Cordis |
Biotroniks |
Biocompati-bles |
Biocompati-bles |
BioDiamond |
Stent type |
ACS Multilink |
NIR |
Palmaz-Shatz |
Tenax |
BiodivYsio |
BiodivYsio |
DLC |
Study |
West |
Finess I |
Benestent I |
Tenax Study, NL |
SOPHOS A |
SOPHOS B |
Barragan |
Nr. Patients |
102 |
255 |
259 |
174 |
200 |
226 |
140 |
Nr. stents |
193 |
207 |
|||||
Stent |
15 |
9,16,32 |
15,25,35 |
10,15,25 |
15,28 |
9,26,25 |
|
Stent |
2.5,3,3.5 |
||||||
Sub-acute |
0.80% |
||||||
Death |
3.10% |
0.80% |
2.80% |
0.50% |
1.30% |
0.00% |
|
MI |
6.00% |
5.80% |
4.20% |
6.30% |
5.00% |
3.60% |
0.70% |
CABG |
** |
5.10% |
5.00% |
** |
2.50% |
0.90% |
1.50% |
Re-PTCA |
18.00% |
9.40% |
10.00% |
15.00% |
8.00% |
5.30% |
6.70% |
MACE |
23.00% |
23.50% |
20.10% |
24.90% |
16.00% |
11.10% |
8.90% |
MI: myocardial infarction
CABG: coronary artery bypass grafting
PTCA: percutaneous transluminal coronary angioplasty
MACE: major adverse cardiac events, MI + CABG + PTCA
**: CABG and re-PTCA were not separately listed
TABLE 2: Patient history
Company |
Biocompatibles |
BioDiamond |
Stent |
BiodivYsio |
DLC |
Study |
SOPHOS A/B |
Barragan |
Patient Nr |
426 |
140 |
Age |
60 ± 10 years |
60 ± 10 years |
Male |
74.20% |
77.00% |
Prior MI |
31.30% |
23.00% |
Prior CABG |
2.60% |
12.00% |
Prior PTCA |
11.80% |
25.00% |
TABLE 3: Angiographic data of stented lesions in the Barragan study
142 patients with 212 lesions |
|
Lesion catagory (A to C) |
|
A |
25% |
B1 |
22% |
B2 |
25% |
C |
28% |
One vessel disease |
34% |
Two vessel disease |
38% |
Three vessel disease |
28% |
TABLE 4: Patient history and target lesion revascularisation (TLR) after 6 months in the 1st Barragan study
Reason for stenting |
with TLR |
without TLR |
MACE rate |
|
n=17 |
n=180 |
|
In-Stent Restenosis |
5 |
5 |
50.00% |
Restenosis |
1 |
7 |
12.50% |
Total Chronic Occlusion |
3 |
3 |
50.00% |
SVG |
1 |
3 |
25.00% |
Acute MI |
1 |
17 |
|
De novo lesion |
6 |
155 |
3.70% |
This rate was approved in second study (523 stentings) reported at conference in Marseille in April 2000. This second study brought also new very promising fact, namely practically independence of TVR on the length of stent used (no difference between 9 mm and 16 mm stents). For other commercial products the proportional growth of TVR with increased length of stent was observed and presents well-known fact. This important observation gives evidence that surface of implant, which is increased with increased stent length, does not bring negative influence in case of BioDiamond coated stents in contrast to stainless steel surface.
Generally speaking BioDiamond stents, without use of any cytotoxic/cytostatic agent, demonstrate less than 4% restenosis for de novo lesions and less than 10% for non-selected patients. It means, that BioDiamond implants have the same or even better effectiveness as the most modern drug eluting stents (such as Cordis/Cypher, study SIRIUS: MACE ca. 10% at present time point with tendency for further growth) at the market.
For further details please revert to the attached data of studies.
7. Ability of small vessels stenting
The sum of above positive features in combination with new special design allowed to develop new product - "BioDiamond micro" stent for stenting of small (2.0 and 2.5 mm vessels). For more details please see attached Description of "BioDiamond micro" stent.
References about BioDiamond Stents:
1. P. Barragan et al., "The BioDiamond and BioDiamond F stents", in: "Handbook of Coronary Stents, 3rd Edition", Editors P.W. Serruys and M.J.B. Kutryk, Martin Dunitz, 2000, p. 29-39
2. K. Gutensohn et al., "In vitro Biocompatibility Analyses of Stents Coated with Diamond-Like Carbon by Flow Cytometry, Cell Growth Assays and Electron Microscopy", Inf.Ther.Transf. Med., No.4, v.27, 2000, p. 200-205
3. K. Gutensohn et al., "In vitro Analyses of Diamond-Like Carbon coated Stents: Reduction of Metal Ion Release, Platelet Activation, and Thrombogenecity", Thromb. Res., v. 99, No. 6, 2000, p. 577-585
4. T.A. Batyraliev et al., "Immediate and long-term effects of coronary stent BioDiamond implantation", Terapevticheskii Arkhiv, 2002, No.2, p. 57-60
